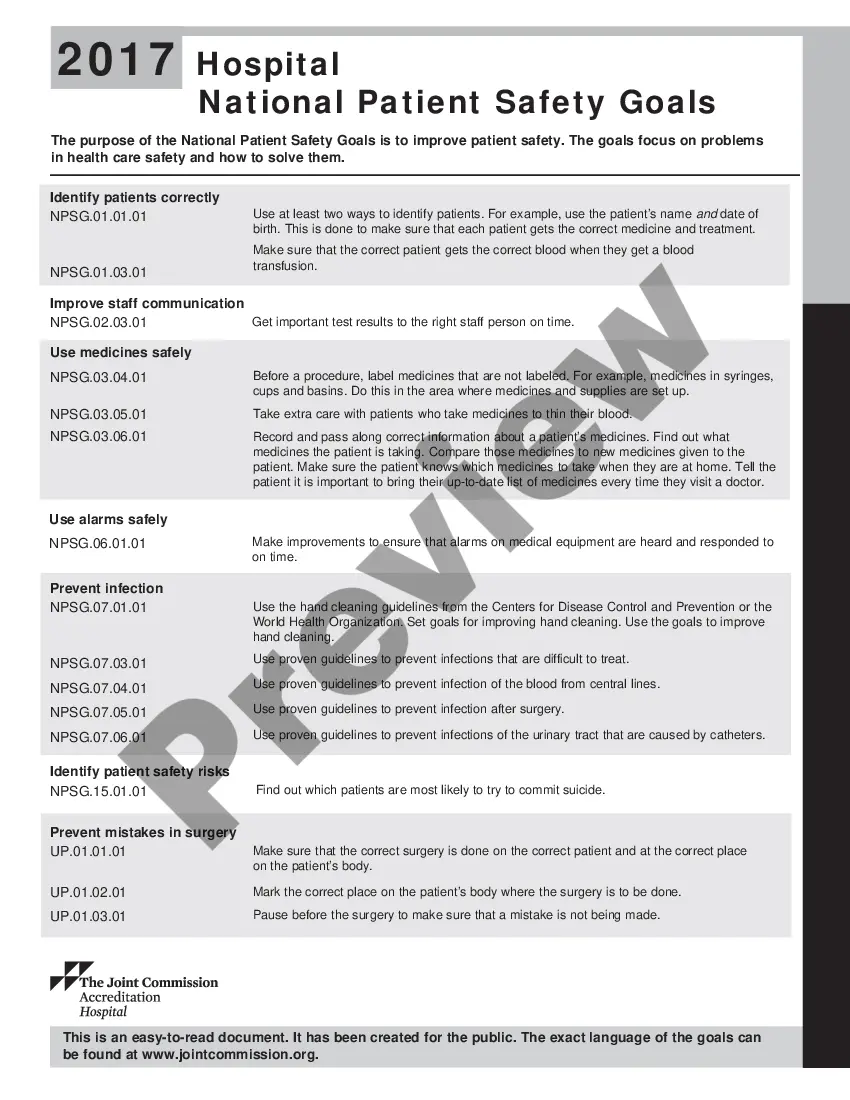
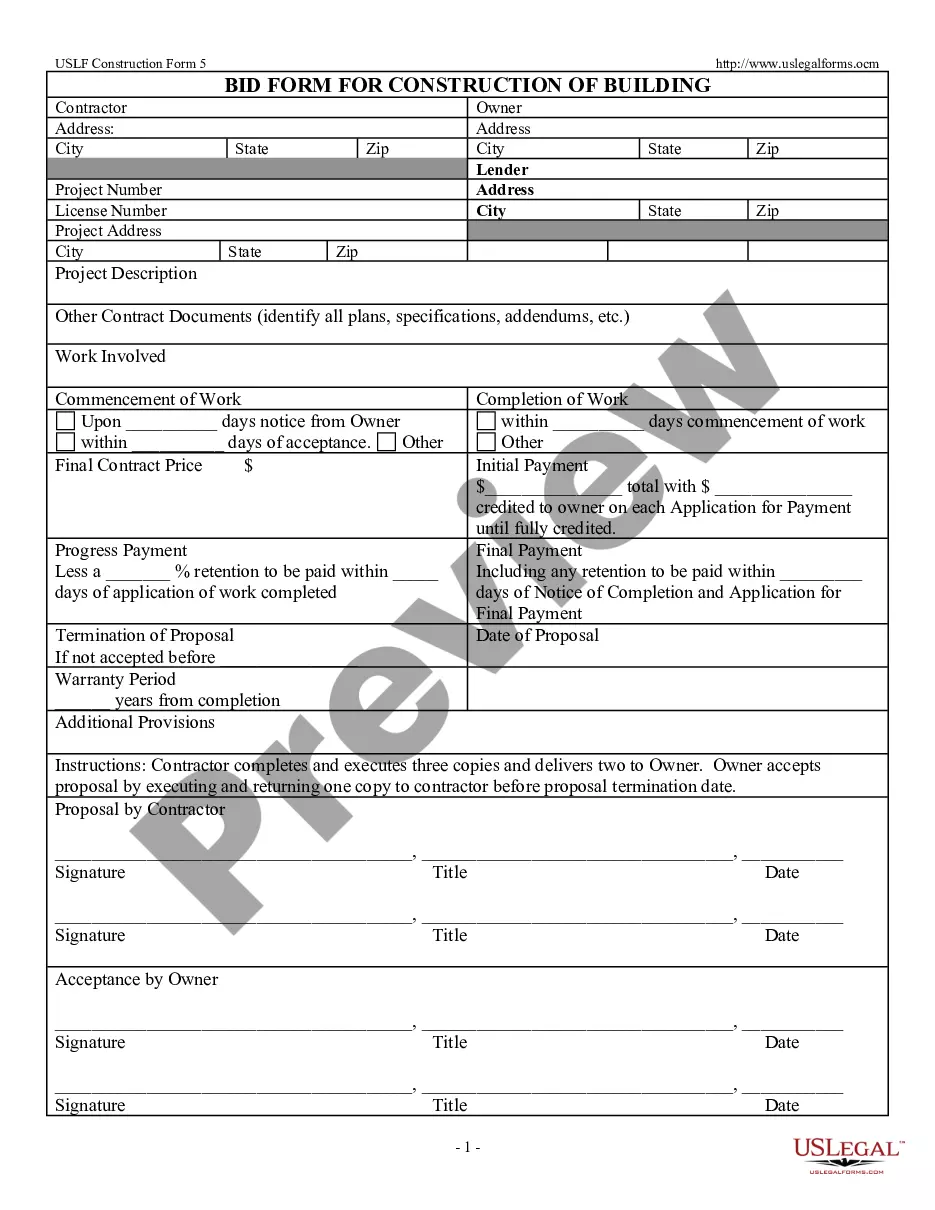
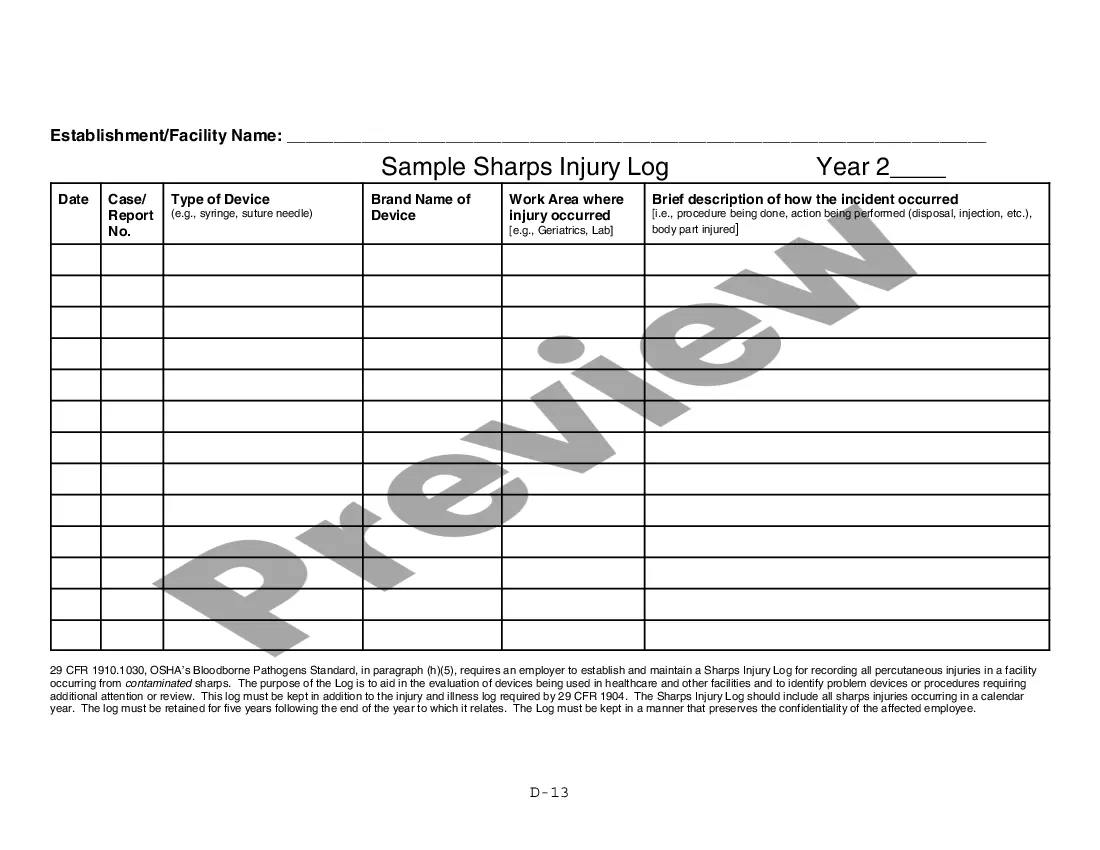
Georgia Aeseptic Techniques
Description
How to fill out Aeseptic Techniques?
US Legal Forms - one of the largest collections of legal documents in the USA - offers a broad array of legal document templates that you can download or create.
By utilizing the website, you can obtain thousands of forms for business and personal use, organized by categories, states, or keywords. You can find the latest forms such as the Georgia Aseptic Techniques within moments.
If you already possess a monthly subscription, Log In and download Georgia Aseptic Techniques from the US Legal Forms library. The Download button will appear on each form you view. You can access all previously acquired forms in the My documents section of your account.
Complete the purchase. Use your credit card or PayPal account to finalize the transaction.
Select the format and download the form to your device. Make modifications. Fill out, edit, print, and sign the downloaded Georgia Aseptic Techniques. Each template you add to your account has no expiration date and is yours permanently. Therefore, if you wish to download or print another copy, just visit the My documents area and click on the form you need.
Access the Georgia Aseptic Techniques with US Legal Forms, the most extensive collection of legal document templates. Utilize thousands of professional and state-specific templates that fulfill your business or personal needs and requirements.
- Ensure you have chosen the correct form for your city/county.
- Click the Review button to examine the form's content.
- Read the form description to ensure you have selected the right form.
- If the form does not meet your requirements, use the Search box at the top of the screen to find one that does.
- If you are satisfied with the form, confirm your choice by clicking the Buy now button.
- Then, choose the pricing plan you prefer and provide your details to register for an account.
Form popularity
FAQ
Principles of Sterile TechniqueFace to face or back to back.Turn back to a non-sterile person or when passing.Face a sterile area when passing the area.Ask a non-sterile person to step aside rather than trying to crowd past him.Step back away from the sterile field to sneeze or cough.More items...?
PRINCIPLES OF THE ASEPTIC TECHNIQUECreating a microorganism-free environment (sterile field)Use of sterilized instruments and dressings.Maintaining sterility of sterile field and instruments by preventing microbial contaminationby contact with non-sterile objects; such as:More items...
According to The Joint Commission, there are four chief aspects of the aseptic technique: barriers, patient equipment and preparation, environmental controls, and contact guidelines. Each plays an important role in infection prevention during a medical procedure.
Aseptic technique is a collection of medical practices and procedures that helps protect patients from dangerous germs. Bacteria, viruses, and microorganisms are everywhere, so using aseptic technique can help keep important equipment from being contaminated.
Aseptic techniques include:Wiping bench with disinfectant/alcohol. Not growing microorganisms at body temperature. Using sterile loops when transferring cultures . Flaming culture bottle necks to prevent contamination. Sterilising (using an autoclave ) or disposing of all used equipment.
These principles include the following: (1) use only sterile items within a sterile field; (2) sterile (scrubbed) personnel are gowned and gloved; (3) sterile personnel operate within a sterile field (sterile personnel touch only sterile items or areas, unsterile personnel touch only unsterile items or areas); (4)
Procedures that involve aseptic technique include:200cInserting PICC lines.200cPerforming dialysis.200cInserting catheters.200cRunning IVs.200cInserting chest tubes.200cPerforming surgeries.200cDressing wounds.
Aseptic technique is classified into two different categories: standard aseptic technique and surgical aseptic technique.