Hennepin Minnesota Employment Agreement — Clinical Research Coordinator and Clinical Trial Firm Introduction: The Hennepin Minnesota Employment Agreement for a Clinical Research Coordinator (CRC) and Clinical Trial Firm is a legally binding contract that outlines the terms and conditions of employment between the CRC and the clinical trial firm located in Hennepin, Minnesota. This agreement is specifically tailored to the role of a CRC within the clinical trial industry in Hennepin County. Key Terms and Conditions: 1. Job Description: This agreement includes a detailed description of the CRC's responsibilities, such as recruiting patients, coordinating clinical trials, maintaining trial documentation, ensuring compliance with regulations, and collaborating with other healthcare professionals. 2. Compensation: The agreement outlines the CRC's salary, potential bonuses, overtime rates, and any additional benefits such as healthcare, retirement plans, or vacation days. 3. Duration and Termination: It specifies the duration of employment, whether it is a fixed-term contract or an open-ended agreement. The termination clauses detail circumstances under which either party can terminate the agreement and the notice period required. 4. Confidentiality and Intellectual Property: The agreement emphasizes the importance of maintaining patient confidentiality and protecting intellectual property rights related to the clinical trials conducted by the firm. 5. Non-Competition and Non-Solicitation: This section restricts the CRC from engaging in activities that may directly or indirectly compete with the clinical trial firm or solicit clients or employees while employed and after termination. Types of Hennepin Minnesota Employment Agreement — Clinical Research Coordinator and Clinical Trial Firm: 1. Full-Time Employment Agreement: This is the most common type of agreement where the CRC is contracted for a fixed number of hours per week, typically 40 hours, and receives all associated benefits. 2. Part-Time Employment Agreement: This agreement is suitable for Arcs who work fewer hours, often less than 40 hours per week, and may receive prorated benefits. 3. Consultant Agreement: Sometimes, clinical trial firms hire Arcs as independent consultants for specific projects. This agreement is designed for such arrangements, usually for a fixed duration and specific project goals. 4. Fixed-Term Agreement: This type of agreement is used when the CRC is contracted for a predetermined period, typically when working on time-limited clinical trials or research projects. Conclusion: The Hennepin Minnesota Employment Agreement for a Clinical Research Coordinator and Clinical Trial Firm serves as a comprehensive document that ensures transparency and clarity in the employment relationship between the CRC and the clinical trial firm. By outlining job responsibilities, compensation, termination conditions, and other key terms, this agreement promotes a fair and mutually beneficial working arrangement that upholds legal requirements, protects confidentiality, and encourages successful clinical trial operations.
Para su conveniencia, debajo del texto en español le brindamos la versión completa de este formulario en inglés. For your convenience, the complete English version of this form is attached below the Spanish version.Hennepin Minnesota Acuerdo Laboral - Coordinador de Investigación Clínica y Firma de Ensayos Clínicos - Employment Agreement - Clinical Research Coordinator and Clinical Trial Firm
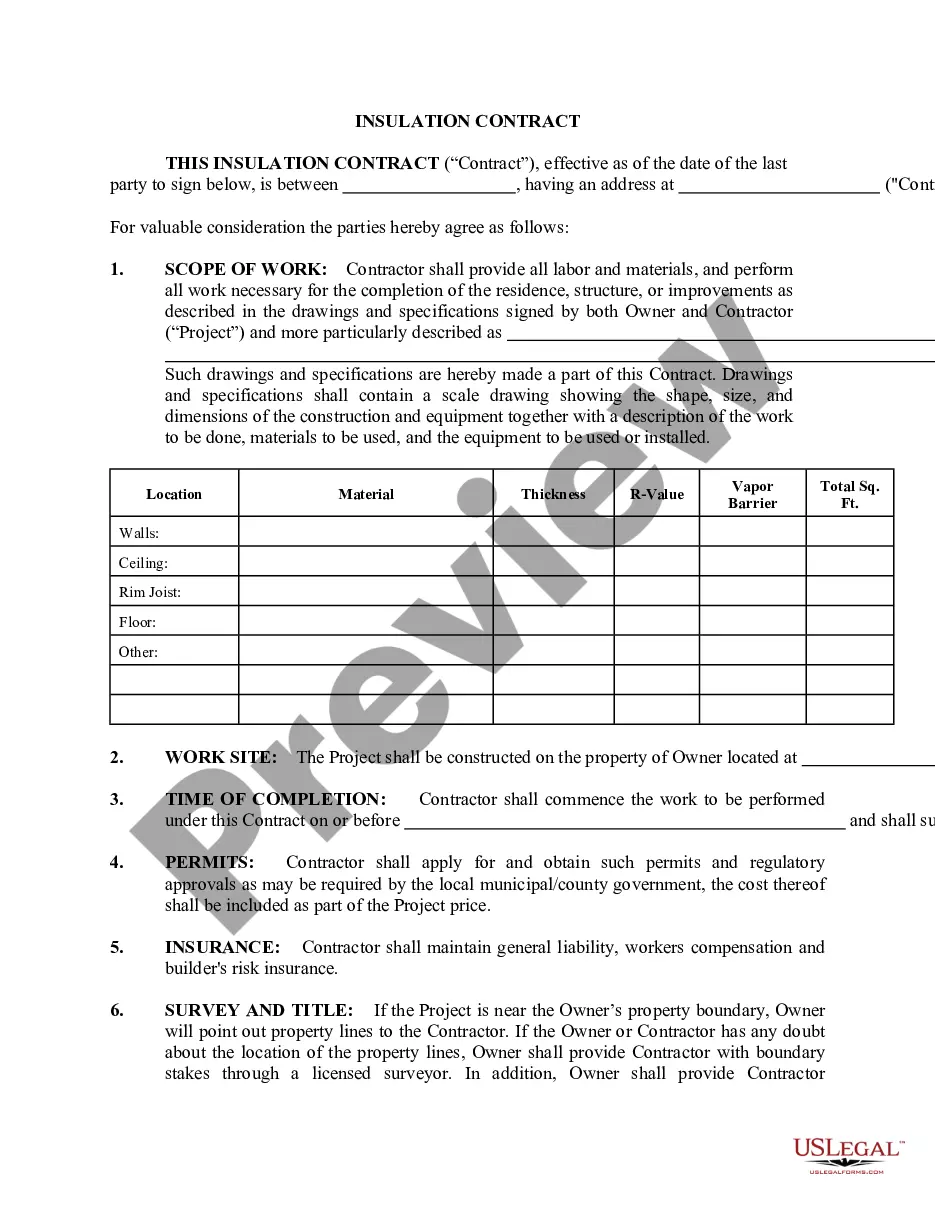
Description
How to fill out Hennepin Minnesota Acuerdo Laboral - Coordinador De Investigación Clínica Y Firma De Ensayos Clínicos?
Are you looking to quickly create a legally-binding Hennepin Employment Agreement - Clinical Research Coordinator and Clinical Trial Firm or probably any other form to manage your personal or corporate affairs? You can go with two options: contact a legal advisor to draft a valid document for you or create it completely on your own. The good news is, there's an alternative option - US Legal Forms. It will help you get neatly written legal documents without having to pay unreasonable prices for legal services.
US Legal Forms provides a rich collection of over 85,000 state-specific form templates, including Hennepin Employment Agreement - Clinical Research Coordinator and Clinical Trial Firm and form packages. We provide templates for a myriad of use cases: from divorce paperwork to real estate document templates. We've been on the market for over 25 years and got a spotless reputation among our clients. Here's how you can become one of them and obtain the needed template without extra hassles.
- To start with, carefully verify if the Hennepin Employment Agreement - Clinical Research Coordinator and Clinical Trial Firm is adapted to your state's or county's regulations.
- In case the document includes a desciption, make sure to check what it's suitable for.
- Start the searching process again if the form isn’t what you were looking for by utilizing the search bar in the header.
- Select the subscription that is best suited for your needs and move forward to the payment.
- Choose the format you would like to get your document in and download it.
- Print it out, complete it, and sign on the dotted line.
If you've already registered an account, you can easily log in to it, find the Hennepin Employment Agreement - Clinical Research Coordinator and Clinical Trial Firm template, and download it. To re-download the form, just go to the My Forms tab.
It's easy to buy and download legal forms if you use our services. Additionally, the paperwork we offer are updated by industry experts, which gives you greater peace of mind when dealing with legal affairs. Try US Legal Forms now and see for yourself!