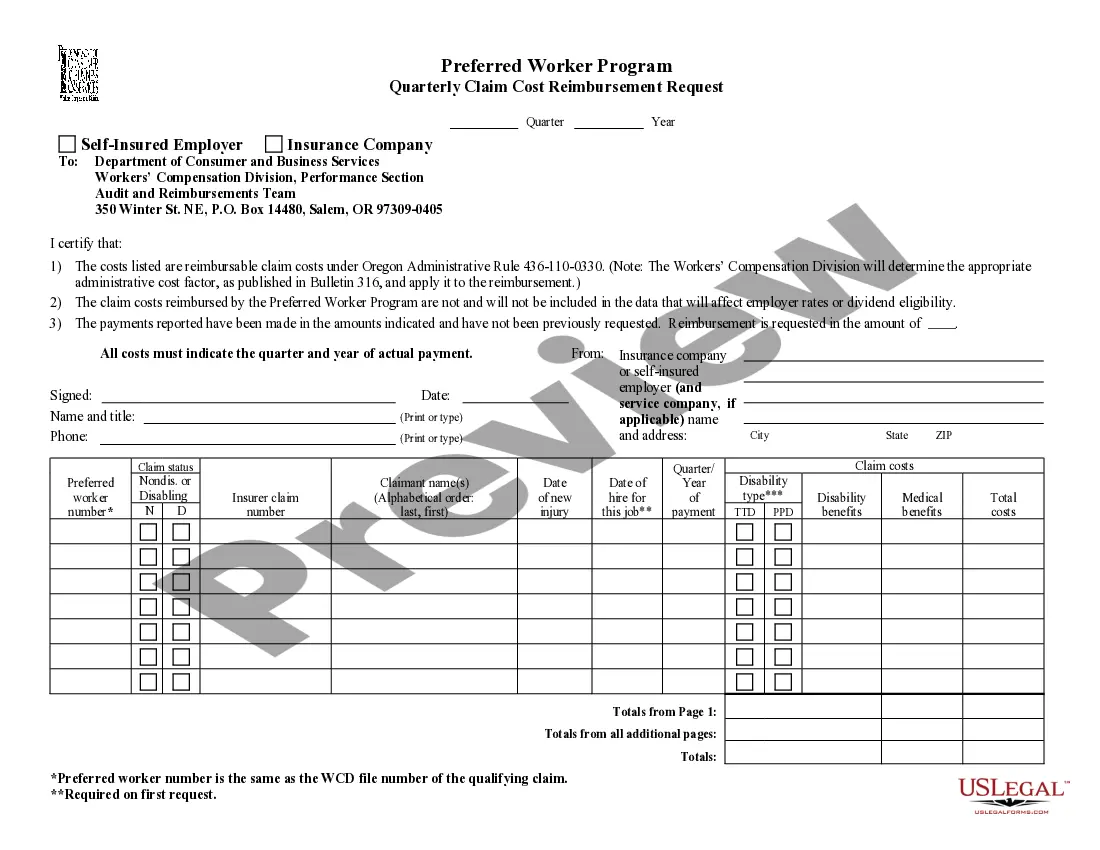
Release Of Information Consent Form Psychology In Clark
Description
Form popularity
FAQ
For an ethically valid consent, information provided to a research subject should include, but not be limited to: information about the health condition for which the research is proposed; details of the nature and purpose of the research; the expected duration of the subject's participation; a detailed description of ...
“Informed Consent, psychologists inform participants about (1) the purpose of the research, expected duration and procedures; (2) their right to decline to participate and to withdraw from the research once participation has begun; (3) the foreseeable consequences of declining or withdrawing; (4) reasonably foreseeable ...
The entire informed consent process involves giving a subject adequate information concerning the study, providing adequate opportunity for the subject to consider all options, responding to the subject's questions, ensuring that the subject has comprehended this information, obtaining the subject's voluntary agreement ...
The elements needed for the documentation of the informed consent discussion include: The nature of the procedure or intervention. The risks and benefits of the procedure or intervention. Reasonable alternatives.
4.3. What are the requirements for obtaining a valid consent? Four core criteria must be met: the patient giving consent must have capacity • the consent must be freely given • the consent must be sufficiently specific to the procedure or treatment proposed • the consent must be informed.
Following are some legally required elements of informed consent: At the beginning of each consent form: a statement of key information that a reasonable person would want to have in order to understand the reasons why one might or might not want to participate in the research.
Using this strategy, legal, regulatory, philosophical, medical, and psychological literatures have come together to support the following elements of informed consent: (1) disclosure, (2) understanding, (3) voluntariness, (4) competence, and (5) consent (see National Commission 1978; Meisel and Roth 1981; President's ...
At a minimum, a well-designed informed consent form will address the following information: Risks and benefits of treatment. Fees and payment policies. Confidentiality and its limits. Contact information and communication. Social media policy and general boundaries. Emergency procedures.
Basic Elements of Informed Consent Purpose of the Research. Description of the Research. Risks. Benefits. Alternatives to Participation.
The five key elements of consent are: the individual gives consent voluntarily. the individual is adequately informed before giving consent. the consent is specific. the consent is current. the individual has the capacity to understand and communicate their consent.