Louisiana Authorization to Use or Disclose Protected Health Information
Description
How to fill out Authorization To Use Or Disclose Protected Health Information?
You might spend time online seeking the legal document format that meets the federal and state criteria you require.
US Legal Forms offers a vast array of legal templates that are reviewed by experts.
You can download or print the Louisiana Authorization to Use or Disclose Protected Health Information from the service.
First, confirm that you have selected the correct format for your county/region of choice. Review the form description to ensure you have chosen the correct document. If available, use the Review option to browse through the format as well.
- If you already have a US Legal Forms account, you may sign in and click on the Obtain option.
- Afterward, you can complete, modify, print, or sign the Louisiana Authorization to Use or Disclose Protected Health Information.
- Every legal document template you purchase is yours indefinitely.
- To acquire another copy of any purchased form, visit the My documents tab and select the relevant option.
- If you're using the US Legal Forms website for the first time, follow the simple instructions below.
Form popularity
FAQ
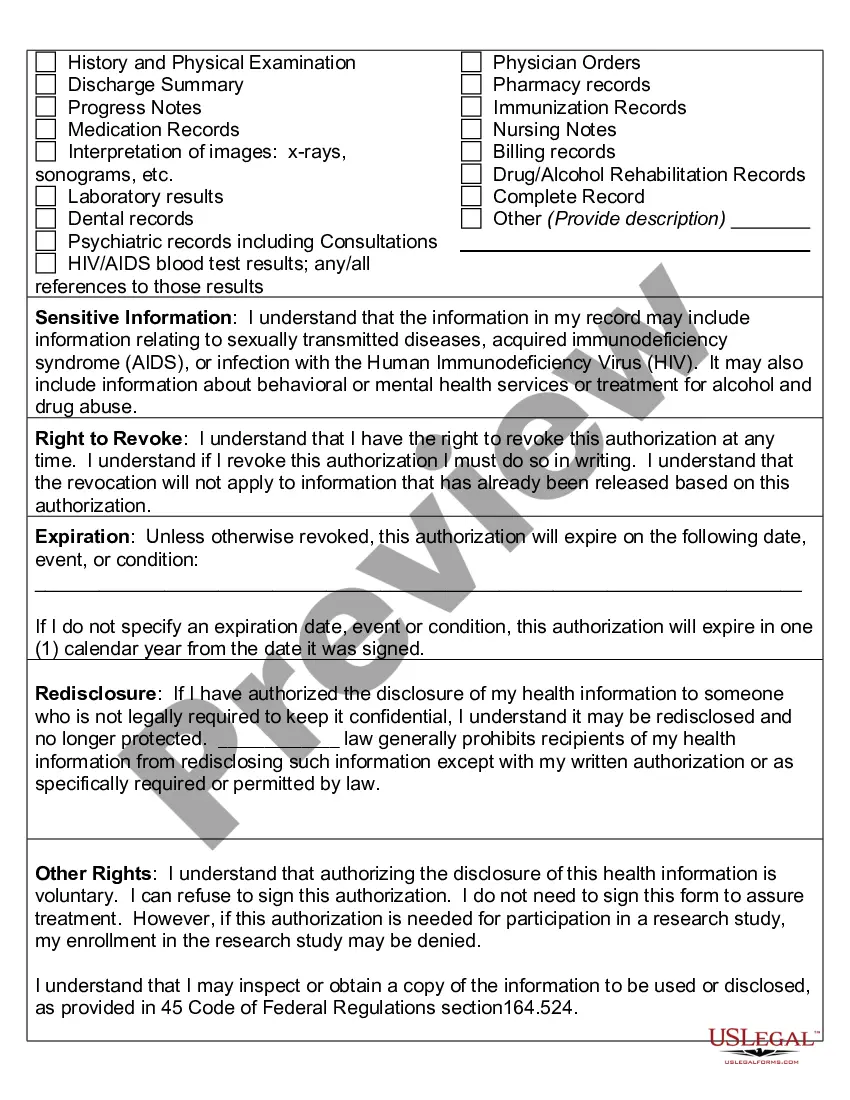
An authorization must specify a number of elements, including a description of the protected health information to be used and disclosed, the person authorized to make the use or disclosure, the person to whom the covered entity may make the disclosure, an expiration date, and, in some cases, the purpose for which the
The HIPAA Privacy Rule requires that an individual provide signed authorization to a covered entity, before the entity may use or disclose certain protected health information (PHI).
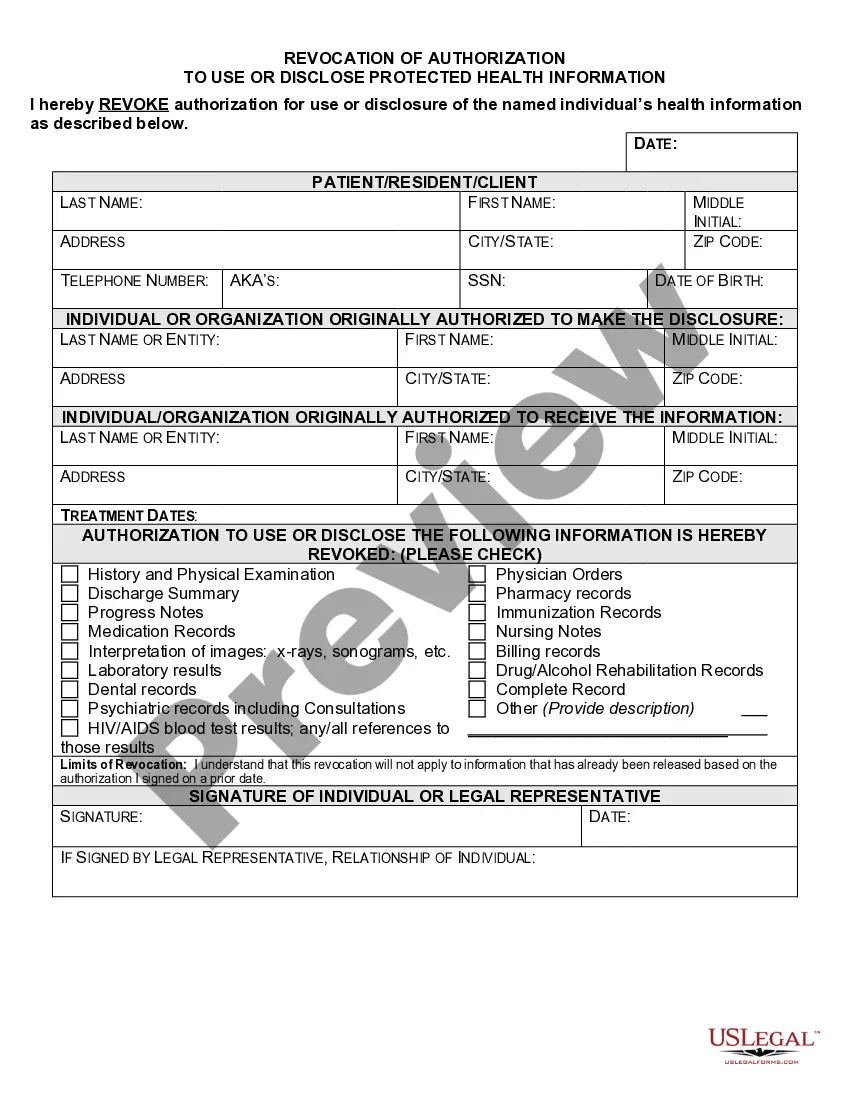
What are two required elements of an authorization needed to disclose PHI? Response Feedback: All authorizations to disclose PHI must have an expiration date and provide an avenue for the patient to revoke his or her authorization. What does the term "Disclosure" mean?
A covered entity must obtain the individual's written authorization for any use or disclosure of protected health information that is not for treatment, payment or health care operations or otherwise permitted or required by the Privacy Rule.
Research: An authorization for the use or disclosure of PHI for a research study may be combined with any other type of written permission for the same or another research study, including a consent to participate in the research or another authorization to disclose protected health information from the research.
Generally, your PHI may be used and disclosed by us only with your express written authorization. However, there are some exceptions to this general rule. Treatment Purposes. We may use or disclose your PHI to provide, coordinate, or manage your medical treatment or services.
A patient authorization is not required for disclosure of PHI between Covered Entities if the disclosure is needed for purposes of treatment or payment or for healthcare operations. You may disclose the PHI as long as you receive a request in writing.
Valid HIPAA Authorizations: A ChecklistNo Compound Authorizations. The authorization may not be combined with any other document such as a consent for treatment.Core Elements.Required Statements.Marketing or Sale of PHI.Completed in Full.Written in Plain Language.Give the Patient a Copy.Retain the Authorization.
A HIPAA authorization is a detailed document in which specific uses and disclosures of protected health are explained in full. By signing the authorization, an individual is giving consent to have their health information used or disclosed for the reasons stated on the authorization.
Under the HIPAA Privacy Rule, a covered entity must disclose protected health information in only two situations: (a) to individuals (or their personal representatives) specifically when they request access to, or an accounting of disclosures of, their protected health information; and (b) to the Department of Health