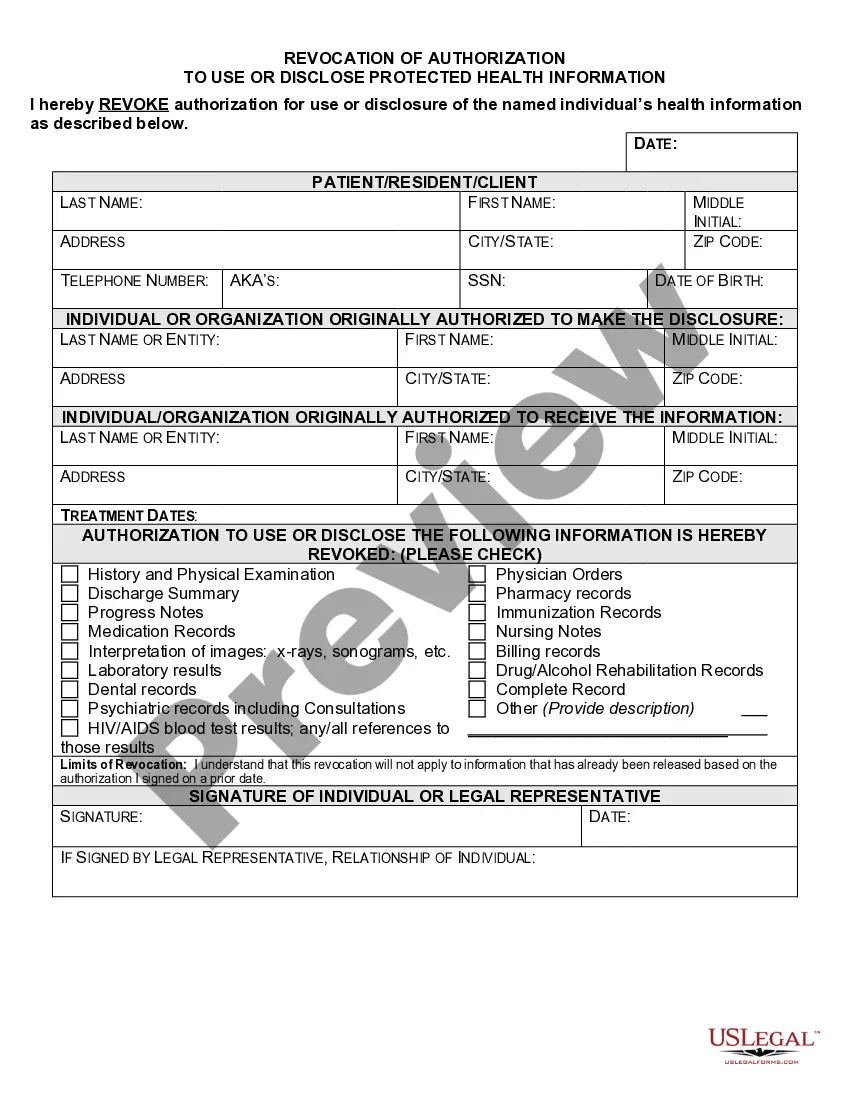
New Mexico Revocation of Authorization To Use or Disclose Protected Health Information
Description
How to fill out Revocation Of Authorization To Use Or Disclose Protected Health Information?
You can spend time on the internet trying to locate the legal document template that meets the state and federal standards you require.
US Legal Forms offers thousands of legal templates that are evaluated by experts.
You can easily download or print the New Mexico Revocation of Authorization To Use or Disclose Protected Health Information from our platform.
If available, take advantage of the Preview button to browse through the document template as well.
- If you already possess a US Legal Forms account, you may Log In and click on the Obtain button.
- Afterwards, you can complete, modify, print, or sign the New Mexico Revocation of Authorization To Use or Disclose Protected Health Information.
- Each legal document template you purchase is yours permanently.
- To obtain an additional copy of any acquired form, visit the My documents section and click on the corresponding button.
- If you are using the US Legal Forms website for the first time, follow the simple instructions below.
- First, make sure you have selected the correct document template for the region/city you choose.
- Review the form description to ensure you have selected the appropriate template.
Form popularity
FAQ
Revoking Consent in Writing However, a patient can also revoke consent through a simple letter revoking all consent given when they first signed the form. It would be helpful for the patient to have a copy of the healthcare provider's HIPAA policy form and a copy of the consent they originally provided.
The HIPAA Privacy Rule requires that an individual provide signed authorization to a covered entity, before the entity may use or disclose certain protected health information (PHI).
Generally, your PHI may be used and disclosed by us only with your express written authorization. However, there are some exceptions to this general rule. Treatment Purposes. We may use or disclose your PHI to provide, coordinate, or manage your medical treatment or services.
An authorization is a detailed document that gives covered entities permission to use protected health information for specified purposes, which are generally other than treatment, payment, or health care operations, or to disclose protected health information to a third party specified by the individual.
Research: An authorization for the use or disclosure of PHI for a research study may be combined with any other type of written permission for the same or another research study, including a consent to participate in the research or another authorization to disclose protected health information from the research.
A research subject may revoke his/her Authorization at any time. The revocation must be in writing. An oral discussion between the subject and member of the research team does not revoke a HIPAA authorization.
Yes. The Privacy Rule gives individuals the right to revoke, at any time, an Authorization they have given.
Call and write the company. Tell the company that you are taking away your permission for the company to take automatic payments out of your bank account. This is called revoking authorization. If you decide to call, be sure to send the letter after you call and keep a copy for your records.
General Authorizations: In accordance with §164.508 of the privacy rule, an authorization for the disclosure of health information may be combined with another authorization. For example, a patient may request lab results be disclosed to two different family members (living in separate residences) on the same form.
To report PHI to law enforcement when required by law to do so (45 CFR 164.512(f)(1)(i)). For example, state laws commonly require health care providers to report incidents of gunshot or stab wounds, or other violent injuries; and the Rule permits disclosures of PHI as necessary to comply with these laws.