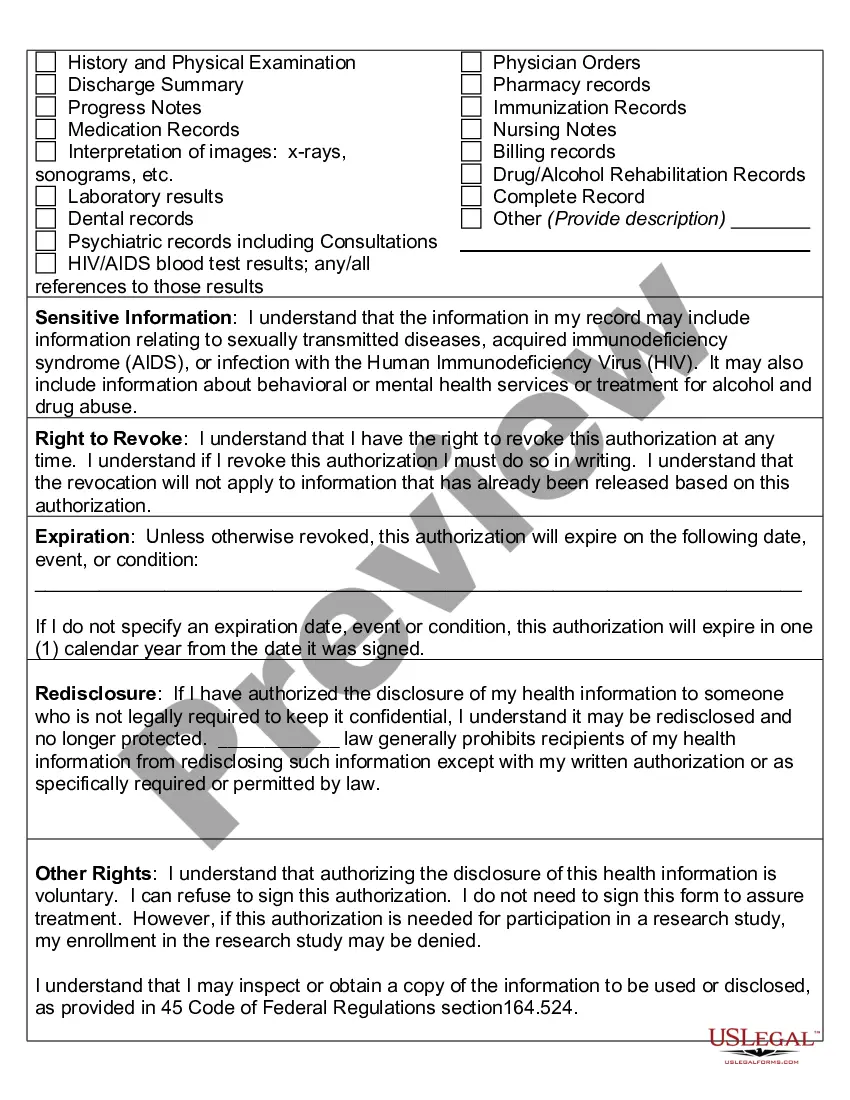
Nevada Authorization to Use or Disclose Protected Health Information: A Detailed Overview In Nevada, the Authorization to Use or Disclose Protected Health Information (PHI) is a crucial document that grants healthcare providers permission to share a patient's sensitive medical information with others for various purposes. This authorization is essential to ensure compliance with federal and state laws, particularly the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. The Nevada Authorization to Use or Disclose PHI is typically obtained from patients when they seek healthcare services or participate in research studies. It provides individuals with control over the dissemination of their protected health information while allowing healthcare providers to collaborate and deliver the appropriate care. Keywords: Nevada, Authorization, Use or Disclose, Protected Health Information, PHI, federal laws, state laws, HIPAA, Privacy Rule, patients, healthcare services, research studies, protected health information, control, collaboration, appropriate care. Different Types of Nevada Authorization to Use or Disclose Protected Health Information: 1. General Authorization: The general authorization grants healthcare providers the ability to disclose and use a patient's protected health information for a broad range of purposes. This may include sharing information with insurance companies for claims processing, consultations with other healthcare professionals, or medical research purposes. 2. Specific Purpose Authorization: A specific purpose authorization is tailored to allow the use or disclosure of PHI for a particular objective. It may be required when a patient wishes to share their medical information for specialized healthcare services like mental health treatment, substance abuse treatment, or HIV/AIDS-related care. 3. Parental/Guardian Authorization: In cases involving minors or individuals incapable of making healthcare decisions for themselves, parental or guardian authorization is necessary. This ensures that parents or legal representatives can access and disclose the protected health information of the dependent individual for proper medical management. 4. Research Authorization: Research authorization is crucial when medical research studies involve the use or disclosure of an individual's protected health information. Researchers must obtain explicit authorization from participants to access their PHI for the purposes of the study. This authorization ensures compliance with ethical and legal guidelines surrounding human subject research. 5. Revocable Authorization: A revocable authorization enables individuals to withdraw their consent for the use or disclosure of their PHI at any time. Patients should be aware of this option and understand the consequences and limitations associated with revoking their authorization. Keywords: General Authorization, Specific Purpose Authorization, Parental/Guardian Authorization, Research Authorization, Revocable Authorization, minors, parental consent, legal representatives, medical research studies, explicit authorization, revoking consent. In conclusion, the Nevada Authorization to Use or Disclose Protected Health Information plays a critical role in safeguarding patients' healthcare privacy while allowing necessary information sharing. Understanding the different types of authorizations available enables healthcare organizations, patients, and researchers to navigate the complex landscape of privacy laws and provide high-quality care and research in compliance with Nevada regulations.
Nevada Authorization to Use or Disclose Protected Health Information
Description
How to fill out Nevada Authorization To Use Or Disclose Protected Health Information?
Have you been within a situation in which you require paperwork for both organization or personal purposes just about every working day? There are a lot of legal record layouts available on the Internet, but finding ones you can rely isn`t effortless. US Legal Forms provides 1000s of develop layouts, much like the Nevada Authorization to Use or Disclose Protected Health Information, which can be published in order to meet state and federal needs.
Should you be already familiar with US Legal Forms website and get a merchant account, merely log in. Following that, it is possible to obtain the Nevada Authorization to Use or Disclose Protected Health Information template.
If you do not come with an accounts and would like to begin using US Legal Forms, abide by these steps:
- Find the develop you want and ensure it is for the correct area/county.
- Make use of the Preview key to examine the form.
- Browse the information to ensure that you have selected the right develop.
- In the event the develop isn`t what you are seeking, utilize the Research industry to find the develop that suits you and needs.
- When you obtain the correct develop, click on Get now.
- Choose the costs strategy you need, submit the specified information to produce your money, and purchase an order using your PayPal or charge card.
- Pick a handy file format and obtain your backup.
Get each of the record layouts you have bought in the My Forms food selection. You can aquire a further backup of Nevada Authorization to Use or Disclose Protected Health Information anytime, if needed. Just click on the required develop to obtain or produce the record template.
Use US Legal Forms, the most considerable assortment of legal forms, to save lots of efforts and stay away from errors. The service provides expertly made legal record layouts that you can use for an array of purposes. Create a merchant account on US Legal Forms and begin making your daily life a little easier.