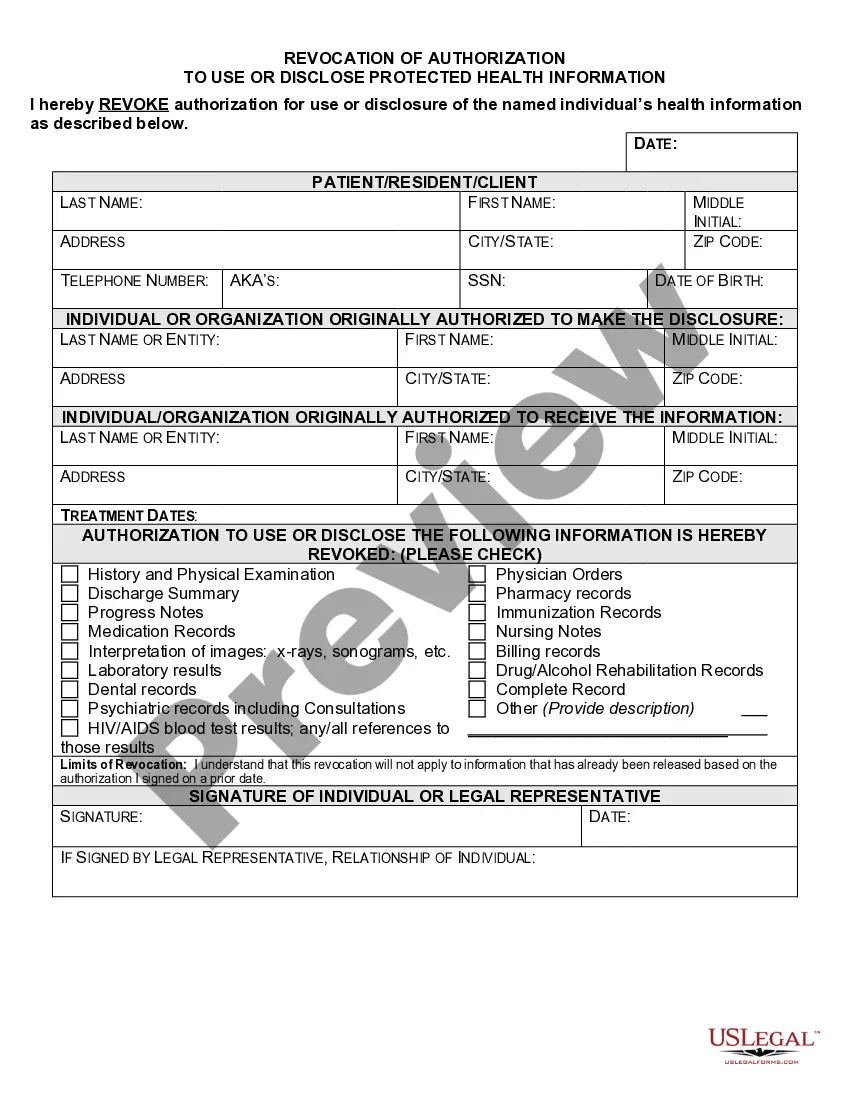
Virginia Authorization for Use and / or Disclosure of Protected Health Information
Description
How to fill out Authorization For Use And / Or Disclosure Of Protected Health Information?
If you wish to finalize, retrieve, or print authentic document formats, utilize US Legal Forms, the most extensive collection of legal templates available online.
Take advantage of the site's user-friendly search feature to locate the documents you require.
Various formats for business and personal use are categorized by types and states, or keywords.
Every legal document template you purchase is your property permanently.
You have access to each form you downloaded in your account. Click the My documents section and select a form to print or download again.
- Employ US Legal Forms to find the Virginia Authorization for Use and/or Disclosure of Protected Health Information with just a few clicks.
- If you are an existing US Legal Forms user, sign in to your account and click the Download button to acquire the Virginia Authorization for Use and/or Disclosure of Protected Health Information.
- Additionally, you can access forms you previously obtained via the My documents section of your account.
- If you are using US Legal Forms for the first time, follow these steps.
- Step 1. Confirm you have selected the form for the correct city/state.
- Step 2. Utilize the Preview option to review the content of the form. Be sure to check the summary.
- Step 3. If you are unsatisfied with the form, use the Search section at the top of the screen to find other versions of the legal document format.
- Step 4. After locating the desired form, click the Buy Now button. Choose your preferred pricing plan and provide your details to register for an account.
- Step 5. Complete the payment process. You can use your Visa, Mastercard, or PayPal account to finalize the transaction.
- Step 6. Select the format of the legal document and download it to your device.
- Step 7. Complete, edit, and print or sign the Virginia Authorization for Use and/or Disclosure of Protected Health Information.
Form popularity
FAQ
An authorization must specify a number of elements, including a description of the protected health information to be used and disclosed, the person authorized to make the use or disclosure, the person to whom the covered entity may make the disclosure, an expiration date, and, in some cases, the purpose for which the
What are two required elements of an authorization needed to disclose PHI? Response Feedback: All authorizations to disclose PHI must have an expiration date and provide an avenue for the patient to revoke his or her authorization. What does the term "Disclosure" mean?
Marketing Activities: A covered entity must obtain an individual's authorization prior to using or disclosing PHI for marketing activities. Marketing is considered any message or statement to the public in an effort to get them to use or seek more information about a product or service.
Valid HIPAA Authorizations: A ChecklistNo Compound Authorizations. The authorization may not be combined with any other document such as a consent for treatment.Core Elements.Required Statements.Marketing or Sale of PHI.Completed in Full.Written in Plain Language.Give the Patient a Copy.Retain the Authorization.
A patient authorization is not required for disclosure of PHI between Covered Entities if the disclosure is needed for purposes of treatment or payment or for healthcare operations. You may disclose the PHI as long as you receive a request in writing.
The HIPAA Privacy Rule requires that an individual provide signed authorization to a covered entity, before the entity may use or disclose certain protected health information (PHI).
Under the HIPAA Privacy Rule, a covered entity must disclose protected health information in only two situations: (a) to individuals (or their personal representatives) specifically when they request access to, or an accounting of disclosures of, their protected health information; and (b) to the Department of Health