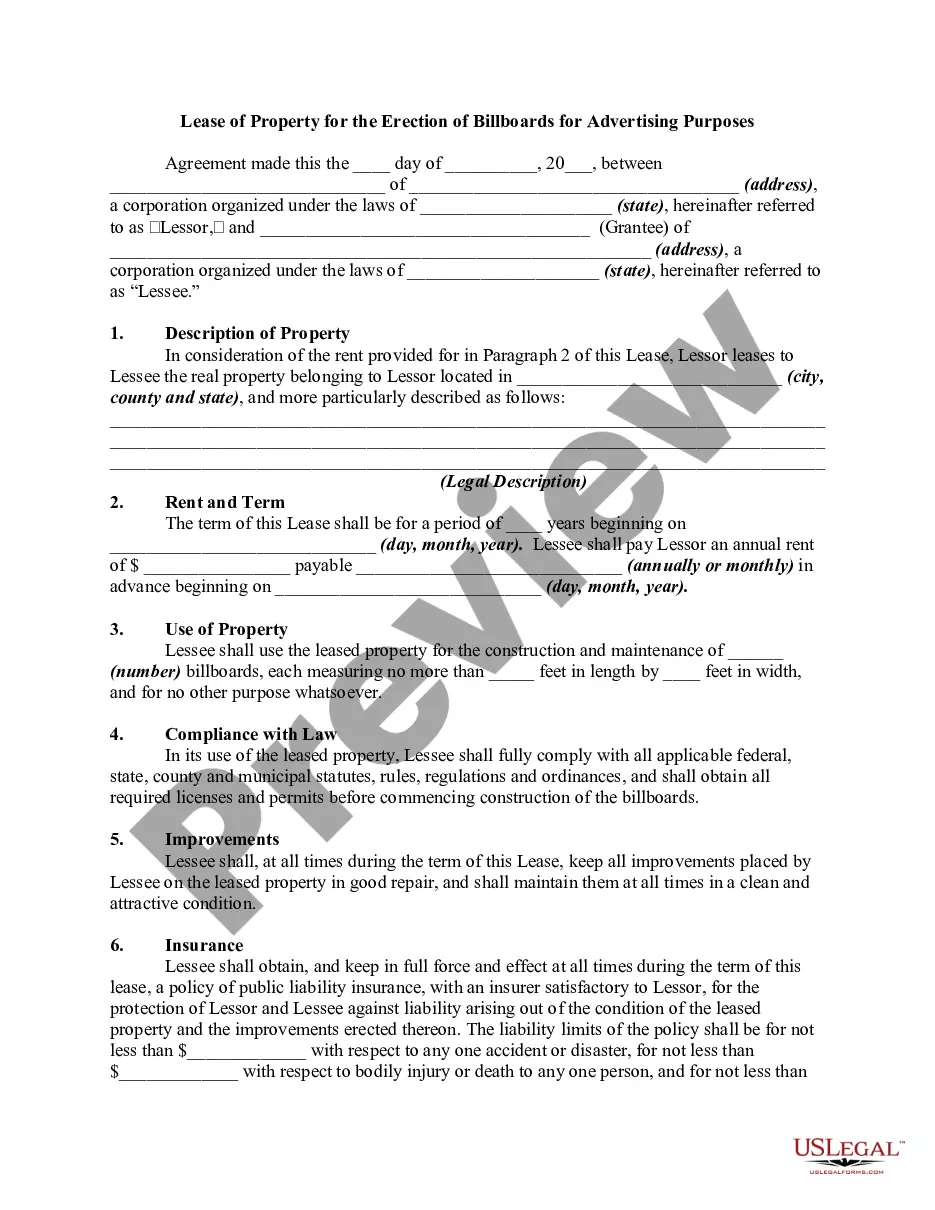
Virgin Islands Release regarding Laboratory Activities is a set of regulations and guidelines established by the Department of Health of the U.S. Virgin Islands. These regulations are in place to ensure the safe and effective operation of laboratories within the Virgin Islands' jurisdiction. Compliance with these guidelines is crucial for laboratory operators, staff, and the overall public health of the region. There are various types of Virgin Islands Release regarding Laboratory Activities, each designed to cover different aspects of laboratory operations. Some key types include: 1. Laboratory Safety Release: This release provides comprehensive guidelines for maintaining a safe work environment within laboratories. It outlines protocols and procedures for handling hazardous materials, disposal of waste, personal protective equipment (PPE) requirements, and emergency response plans. 2. Quality Control Release: This release focuses on ensuring the accuracy and reliability of laboratory test results. It provides guidance on implementing quality control measures, proficiency testing, instrument calibration, and documentation practices maintaining the integrity of data generated by the laboratory. 3. Reporting and Documentation Release: This release details the requirements for properly documenting and reporting laboratory activities. It provides instructions on maintaining accurate records, result reporting formats, and the timely submission of reports to relevant authorities, ensuring compliance with regulatory obligations. 4. Biosafety Release: Laboratories dealing with hazardous biological materials and microorganisms must adhere to the Biosafety Release. This release covers guidelines and protocols related to handling, transport, and storage of infectious agents, biohazard waste management, and personnel training required to prevent the spread of diseases. 5. Research Ethics Release: For laboratories involved in research activities, the Research Ethics Release applies. It defines the ethical principles and standards that researchers must follow when conducting experiments involving human subjects or animals. This release ensures that research protocols align with established ethical guidelines to protect the rights and welfare of participants. Overall, the Virgin Islands Release regarding Laboratory Activities encompasses regulations and guidelines that encompass safety, quality control, reporting, biosafety, and research ethics. Adherence to these releases is integral to maintaining the highest standards of laboratory practice and safeguarding public health in the U.S. Virgin Islands.
Virgin Islands Release regarding Laboratory Activities
Description
How to fill out Virgin Islands Release Regarding Laboratory Activities?
If you want to complete, down load, or print out legal record web templates, use US Legal Forms, the largest collection of legal types, which can be found online. Take advantage of the site`s simple and easy hassle-free research to obtain the papers you require. Different web templates for enterprise and person functions are sorted by categories and suggests, or keywords and phrases. Use US Legal Forms to obtain the Virgin Islands Release regarding Laboratory Activities within a handful of click throughs.
In case you are previously a US Legal Forms buyer, log in in your account and click on the Down load switch to find the Virgin Islands Release regarding Laboratory Activities. You can even entry types you in the past delivered electronically inside the My Forms tab of your account.
If you use US Legal Forms initially, refer to the instructions below:
- Step 1. Make sure you have selected the form for the appropriate city/nation.
- Step 2. Utilize the Review method to examine the form`s articles. Never forget about to learn the description.
- Step 3. In case you are not satisfied with all the form, use the Research field near the top of the display to discover other versions in the legal form format.
- Step 4. When you have located the form you require, go through the Buy now switch. Pick the rates plan you like and put your references to sign up for an account.
- Step 5. Procedure the deal. You can utilize your Мisa or Ьastercard or PayPal account to perform the deal.
- Step 6. Choose the structure in the legal form and down load it on your gadget.
- Step 7. Comprehensive, edit and print out or sign the Virgin Islands Release regarding Laboratory Activities.
Each and every legal record format you get is your own permanently. You may have acces to each and every form you delivered electronically inside your acccount. Go through the My Forms section and decide on a form to print out or down load again.
Be competitive and down load, and print out the Virgin Islands Release regarding Laboratory Activities with US Legal Forms. There are many specialist and express-distinct types you can use to your enterprise or person needs.