The Washington Statement for Compound Prescription is a set of guidelines issued by the Washington State Board of Pharmacy that are intended to ensure safe and effective compound prescription medications. It consists of four different types: the General Principles, the Quality Assurance Principles, the Patient-Specific Principles, and the Dispensing and Storage Principles. The General Principles provide an overarching framework that guides the practice of compounding. These principles include: that the safety and efficacy of the compound must be established, that the compounding must be performed in accordance with appropriate standards of practice, and that the Board of Pharmacy must be informed of any changes in the compounding process. The Quality Assurance Principles provide a detailed set of guidelines for ensuring the accuracy and reliability of compounded medications. These principles include: that the compounding must be performed in a clean and orderly environment, that the compounding should be performed in accordance with written procedures, and that the compounding should be monitored and reviewed on a regular basis. The Patient-Specific Principles are intended to ensure that the compounding process takes into account the individual needs of the patient. These principles include: that the compounding must be tailored to meet the patient's specific needs, that the compounding must be performed in accordance with the patient's instructions, and that the compounding must be supervised and monitored by a qualified pharmacist. Finally, the Dispensing and Storage Principles provide guidance on how to safely and effectively dispense and store compounded medications. These principles include: that the dispensing must be performed in accordance with appropriate standards of practice, that the storage must be consistent with the storage conditions appropriate for the compounded medication, and that the Board of Pharmacy must be notified of any changes in the dispensing and storage process.
Washington Statement for Compound Prescription
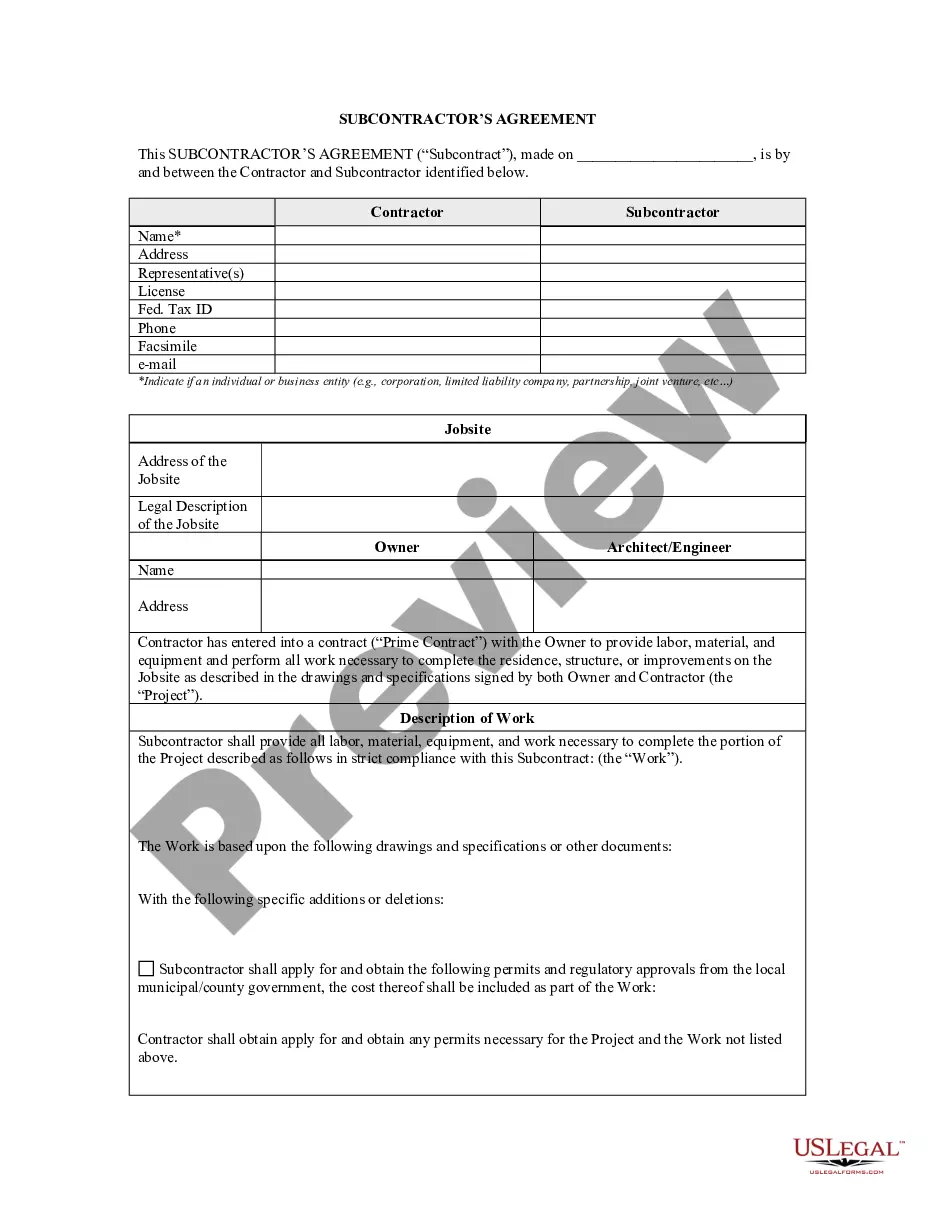
Description
How to fill out Washington Statement For Compound Prescription?
Coping with official paperwork requires attention, precision, and using well-drafted templates. US Legal Forms has been helping people across the country do just that for 25 years, so when you pick your Washington Statement for Compound Prescription template from our service, you can be sure it meets federal and state laws.
Working with our service is straightforward and fast. To get the necessary paperwork, all you’ll need is an account with a valid subscription. Here’s a quick guideline for you to find your Washington Statement for Compound Prescription within minutes:
- Make sure to attentively look through the form content and its correspondence with general and legal requirements by previewing it or reading its description.
- Search for another formal template if the previously opened one doesn’t match your situation or state regulations (the tab for that is on the top page corner).
- Log in to your account and download the Washington Statement for Compound Prescription in the format you need. If it’s your first experience with our website, click Buy now to continue.
- Register for an account, choose your subscription plan, and pay with your credit card or PayPal account.
- Choose in what format you want to save your form and click Download. Print the blank or upload it to a professional PDF editor to prepare it paper-free.
All documents are created for multi-usage, like the Washington Statement for Compound Prescription you see on this page. If you need them in the future, you can fill them out without re-payment - simply open the My Forms tab in your profile and complete your document any time you need it. Try US Legal Forms and accomplish your business and personal paperwork quickly and in total legal compliance!
Form popularity
FAQ
For Physicians: How to Write a Prescription for a Compounded Medication It is important that all prescriptions begin with the title 'Compounded Medication' State that we may compound the prescription. Write the generic name & strength / dose of each API. Choose the dosage form & quantity desired. Provide directions for use.
How to Write a Prescription for a Compounded Medication The generic name of the active ingredients. Strength or dose (in mg or percent) Quantity. Directions for use.
Name of each ingredient used ? NDC (if applicable) of each ingredient used. Quantity of each ingredient used. Ingredient cost of each ingredient used. Total quantity of medication dispensed.
The appropriately trained and competent licensed registered nurse (RN) and licensed practical nurse (LPN) may compound or reconstitute medications for a specific patient as directed by an authorized health care practitioner with prescriptive authority.
What are the four steps to compounding prescriptions? Each individual compounded prescription can be viewed as a four step process: measure, mix, mold, and package.
Examples of medications made by a compounding pharmacy include: Multi-ingredient topical pain creams. Hormone creams, capsules, and suppositories that contain estradiol and progesterone. Rectal suppositories for hemorrhoids or anal fissures.
The information that needs to be documented in a compound record includes name, dose, and strength of the drug; master formulation record information; ingredients; total amount produced; name of all pharmacists involved in the compounding; date; prescription number; label information; and quality control information.
Drug compounding is often regarded as the process of combining, mixing, or altering ingredients to create a medication tailored to the needs of an individual patient. Compounding includes the combining of two or more drugs. Compounded drugs are not FDA-approved.