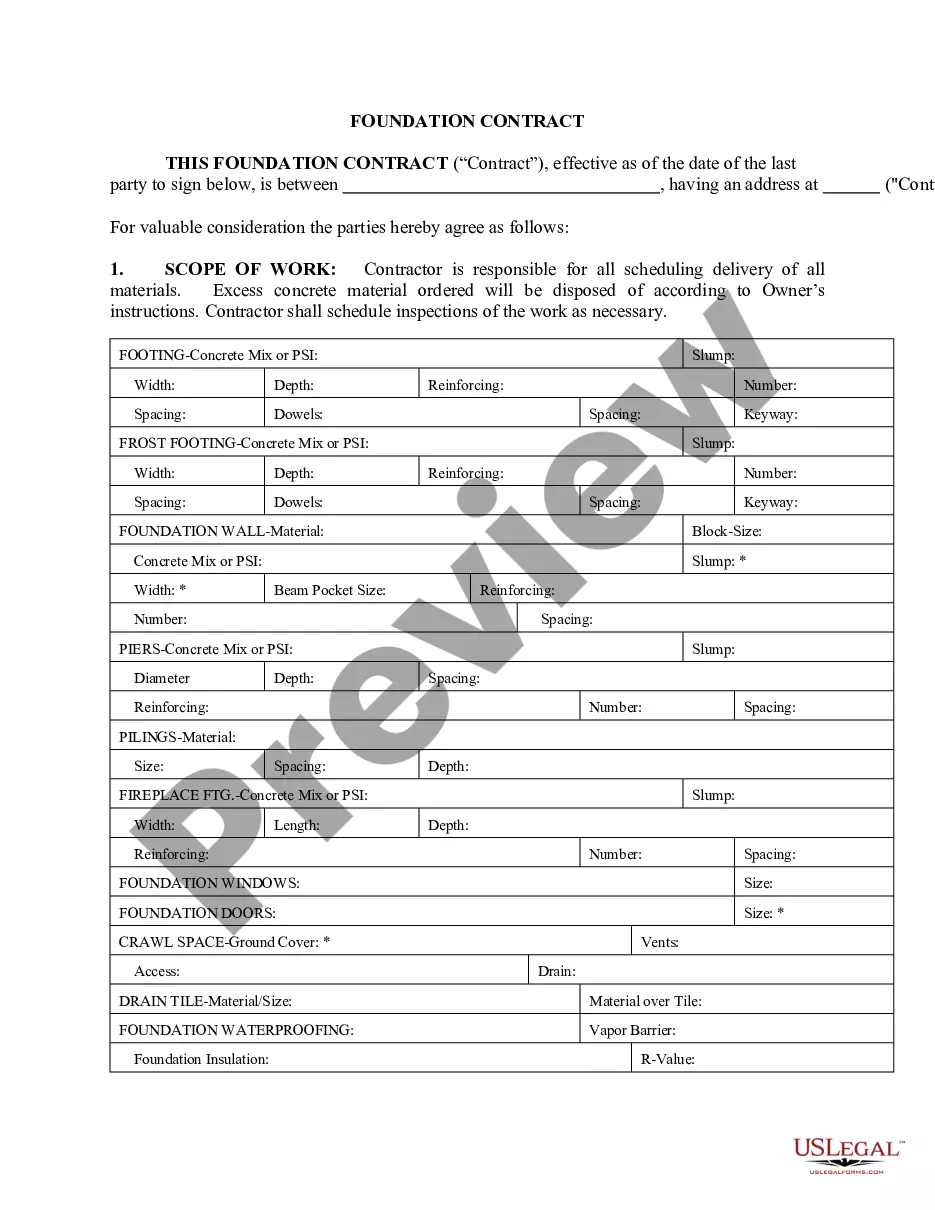
Wayne Michigan Release regarding Laboratory Activities is an official document or agreement that outlines the regulations, guidelines, and procedures associated with laboratory activities within the state of Michigan, specifically in Wayne County. Laboratory activities play a crucial role in various fields such as healthcare, research, and environmental analysis. To ensure the safety and compliance of laboratory operations, the Wayne Michigan Release regarding Laboratory Activities establishes a framework for lab operators, researchers, and technicians to follow. The release covers a wide range of topics and provides detailed information regarding the handling and disposal of hazardous materials, safety protocols, ethical guidelines, and quality control measures. It emphasizes the importance of maintaining a clean and organized laboratory environment to prevent accidents, contamination, and other potential risks. Some key areas covered in the Wayne Michigan Release regarding Laboratory Activities include: 1. Chemical Handling and Storage: This section provides guidelines for the proper storage, labeling, and handling of chemicals used in laboratory activities. It emphasizes the use of safety equipment such as gloves, goggles, and lab coats to minimize exposure to hazardous substances. 2. Biological Safety: Laboratory personnel are instructed on how to handle biological materials safely, including proper disposal methods for biohazardous waste. The release outlines procedures for handling specimens, blood, and other potentially infectious materials to prevent the spread of diseases. 3. Equipment Management: Laboratory instruments and equipment should be calibrated, validated, and maintained regularly to ensure accurate results. This section of the release provides guidelines for maintaining and documenting equipment logs, performing routine maintenance, and troubleshooting common issues. 4. Data Management and Documentation: The release emphasizes the importance of accurate and detailed record-keeping. It outlines procedures for documenting experiments, results, and any deviations from established protocols. Proper data management ensures transparency and reproducibility in scientific studies. 5. Training and Education: Laboratory personnel must undergo regular training and education to stay updated with the latest practices, regulations, and protocols. The release addresses the need for continuous professional development and offers resources for laboratory staff to improve their skills and knowledge. Within the Wayne Michigan Release regarding Laboratory Activities, there may also be separate types or variations based on the specific laboratory setting. For example: 1. Clinical Laboratory Release: This type of release focuses on laboratory activities within clinical settings, such as hospitals or medical diagnostic laboratories. It may include additional guidelines for handling patient samples, complying with HIPAA regulations, and ensuring the accuracy of diagnostic tests. 2. Research Laboratory Release: This variation of the release is tailored for laboratories involved in scientific research, usually located within universities or research institutions. It may emphasize the importance of collaboration, intellectual property rights, and ethical considerations specific to research activities. To adhere to the Wayne Michigan Release regarding Laboratory Activities, laboratory operators and personnel must familiarize themselves with its content and implement the necessary measures to ensure a safe and efficient working environment. Compliance with this release not only protects the well-being of laboratory workers but also contributes to the overall quality and reliability of laboratory results.
Wayne Michigan Release regarding Laboratory Activities
Description
How to fill out Wayne Michigan Release Regarding Laboratory Activities?
Laws and regulations in every area vary throughout the country. If you're not an attorney, it's easy to get lost in a variety of norms when it comes to drafting legal documentation. To avoid expensive legal assistance when preparing the Wayne Release regarding Laboratory Activities, you need a verified template legitimate for your region. That's when using the US Legal Forms platform is so advantageous.
US Legal Forms is a trusted by millions web library of more than 85,000 state-specific legal forms. It's a great solution for professionals and individuals looking for do-it-yourself templates for different life and business occasions. All the documents can be used multiple times: once you obtain a sample, it remains accessible in your profile for future use. Thus, when you have an account with a valid subscription, you can simply log in and re-download the Wayne Release regarding Laboratory Activities from the My Forms tab.
For new users, it's necessary to make several more steps to get the Wayne Release regarding Laboratory Activities:
- Take a look at the page content to ensure you found the correct sample.
- Take advantage of the Preview option or read the form description if available.
- Look for another doc if there are inconsistencies with any of your requirements.
- Click on the Buy Now button to obtain the template when you find the appropriate one.
- Opt for one of the subscription plans and log in or create an account.
- Decide how you prefer to pay for your subscription (with a credit card or PayPal).
- Pick the format you want to save the file in and click Download.
- Complete and sign the template on paper after printing it or do it all electronically.
That's the easiest and most cost-effective way to get up-to-date templates for any legal scenarios. Locate them all in clicks and keep your paperwork in order with the US Legal Forms!