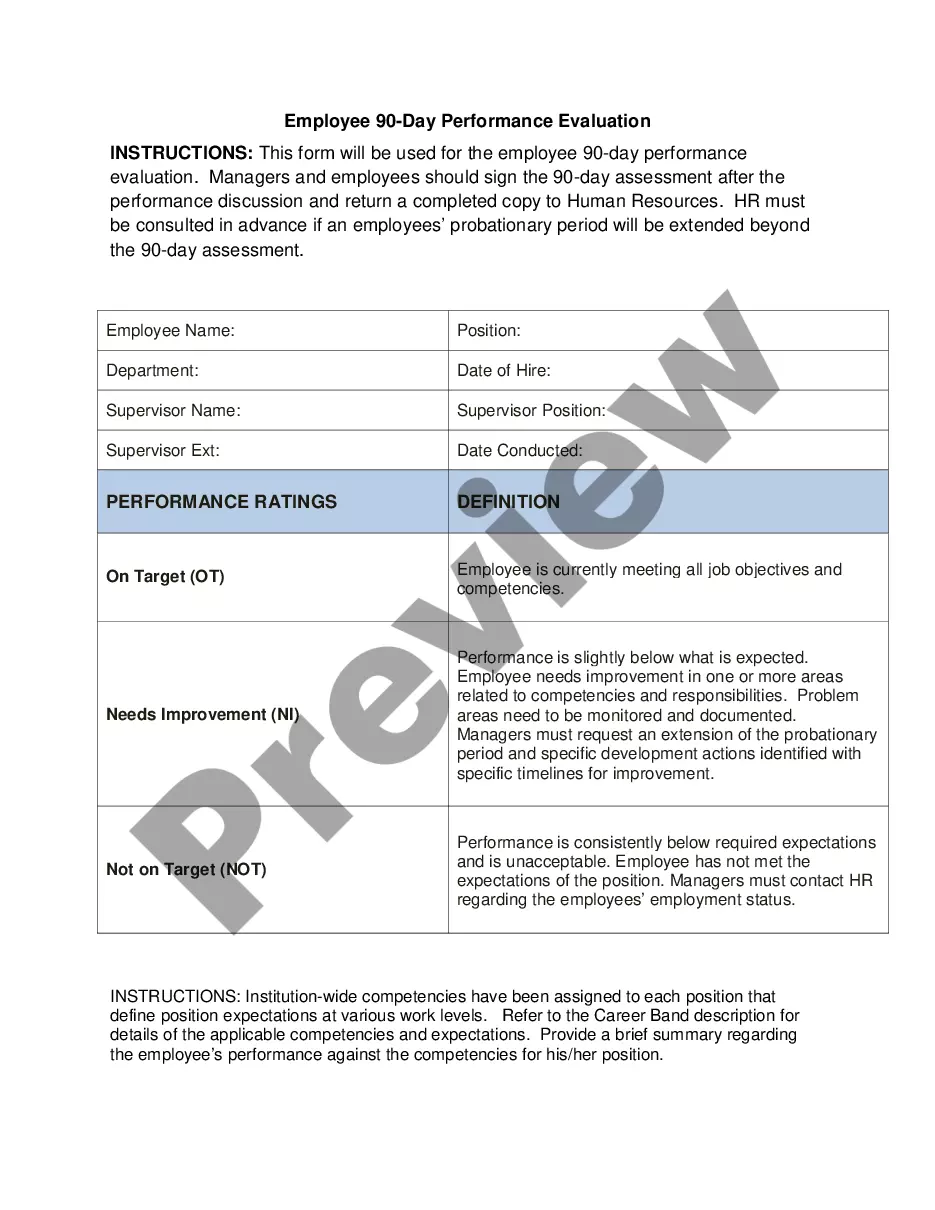
The Santa Clara California Sales, Distribution, and Development Agreement between Supermen, Inc. and Abbott Laboratories is a comprehensive collaboration aimed at accelerating the clinical development process and obtaining necessary regulatory approvals for their innovative pharmaceutical products. This agreement encompasses the following key aspects: 1. Clinical Development: The agreement outlines the joint efforts of Supermen, Inc. and Abbott Laboratories in conducting clinical trials within the Santa Clara, California region. This includes designing and executing studies to evaluate the safety and efficacy of the new pharmaceutical products, often focusing on specific therapeutic areas. 2. Regulatory Approvals: The agreement specifies the shared responsibility to navigate the complex regulatory landscape in order to obtain necessary approvals from regulatory authorities such as the U.S. Food and Drug Administration (FDA). This process involves compiling and submitting comprehensive clinical data, ensuring compliance with regulatory guidelines, and addressing any queries or requests for additional information. 3. Sales and Distribution: The agreement also outlines the terms and conditions for the sales and distribution of the developed pharmaceutical products. Supermen, Inc. and Abbott Laboratories collaborate on marketing strategies, product positioning, pricing, and distribution channels to ensure maximum market penetration and growth potential. 4. Intellectual Property: The agreement includes provisions to protect the intellectual property rights of both parties involved in the collaboration. This ensures that any new scientific discoveries, inventions, or developments resulting from the joint efforts are appropriately protected and utilized. Possible types of Santa Clara California Sales, Distribution, and Development Agreements between Supermen, Inc. and Abbott Laboratories regarding clinical development and regulatory approvals may vary based on specific products or therapeutic areas of focus. These variations could include agreements relating to oncology drugs, infectious disease treatments, cardiovascular medications, or any other specialized pharmaceutical segments. Each agreement would address the unique clinical requirements and regulatory challenges associated with the corresponding therapeutic domain, while reinforcing the overall collaboration between the two companies.
Santa Clara California Sales, Distribution and Development Agreement between Supergen, Inc. and Abbott Laboratories regarding clinical development, obtaining of regulatory approvals
Description
How to fill out Santa Clara California Sales, Distribution And Development Agreement Between Supergen, Inc. And Abbott Laboratories Regarding Clinical Development, Obtaining Of Regulatory Approvals?
Draftwing paperwork, like Santa Clara Sales, Distribution and Development Agreement between Supergen, Inc. and Abbott Laboratories regarding clinical development, obtaining of regulatory approvals, to manage your legal matters is a tough and time-consumming process. Many cases require an attorney’s participation, which also makes this task not really affordable. However, you can get your legal issues into your own hands and deal with them yourself. US Legal Forms is here to save the day. Our website features more than 85,000 legal forms crafted for a variety of scenarios and life circumstances. We make sure each form is compliant with the regulations of each state, so you don’t have to worry about potential legal problems compliance-wise.
If you're already familiar with our services and have a subscription with US, you know how straightforward it is to get the Santa Clara Sales, Distribution and Development Agreement between Supergen, Inc. and Abbott Laboratories regarding clinical development, obtaining of regulatory approvals template. Simply log in to your account, download the template, and personalize it to your needs. Have you lost your form? Don’t worry. You can find it in the My Forms folder in your account - on desktop or mobile.
The onboarding process of new customers is just as easy! Here’s what you need to do before getting Santa Clara Sales, Distribution and Development Agreement between Supergen, Inc. and Abbott Laboratories regarding clinical development, obtaining of regulatory approvals:
- Make sure that your template is compliant with your state/county since the regulations for creating legal papers may vary from one state another.
- Learn more about the form by previewing it or going through a brief description. If the Santa Clara Sales, Distribution and Development Agreement between Supergen, Inc. and Abbott Laboratories regarding clinical development, obtaining of regulatory approvals isn’t something you were looking for, then take advantage of the search bar in the header to find another one.
- Log in or create an account to begin using our website and download the form.
- Everything looks good on your side? Click the Buy now button and choose the subscription plan.
- Pick the payment gateway and type in your payment details.
- Your template is ready to go. You can try and download it.
It’s an easy task to locate and buy the needed document with US Legal Forms. Thousands of businesses and individuals are already taking advantage of our extensive library. Sign up for it now if you want to check what other benefits you can get with US Legal Forms!