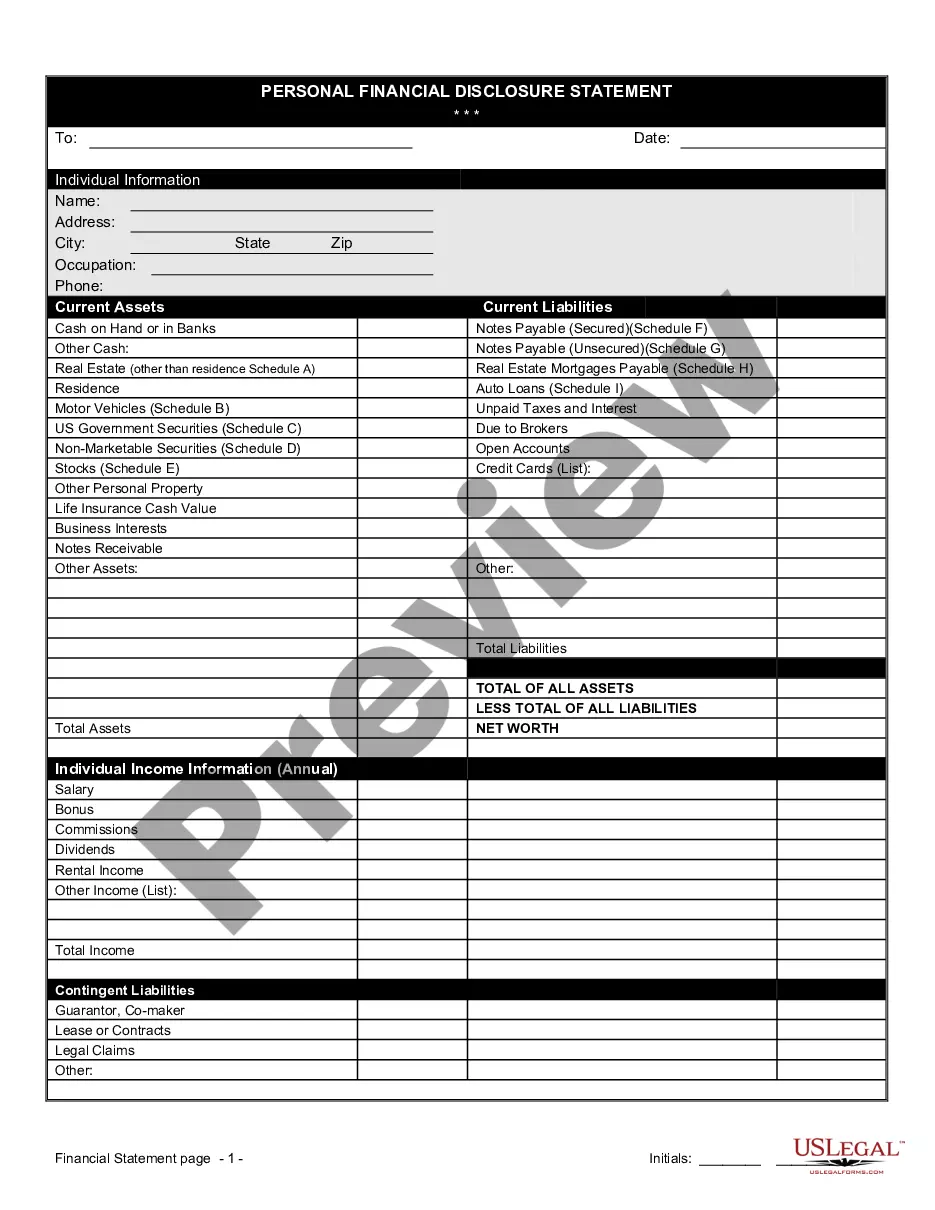
This medical device supply agreement is an agreement that allows the buyer to purchase certain products to be used in the manufacture of its own medical products. The supplier will manufacture, purchase, or assemble the components and sell them to the buyer for use in manufacturing those medical products.
The Contra Costa California Medical Device Supply Agreement is a legally binding contract between a medical device supplier and a healthcare provider in Contra Costa County, California. This agreement outlines the terms and conditions under which the medical devices will be supplied to the healthcare facility. Key Terms and Conditions: 1. Parties involved: The agreement identifies the medical device supplier (company name) and the healthcare provider (hospital, clinic, or healthcare facility) located in Contra Costa County. 2. Definitions: The agreement includes a section that defines the medical devices being supplied, their specifications, and any regulatory requirements that must be adhered to. 3. Scope of supply: This section outlines the specific medical devices to be supplied, including quantity, model numbers, and any additional accessories or spare parts required. It also mentions any limitations or exclusions regarding certain medical devices. 4. Delivery terms: The agreement specifies the terms of delivery, including the delivery location, shipping method, and expected timelines for delivery. It may also outline any penalties or remedies for late or faulty deliveries. 5. Pricing and payment terms: This section details the pricing structure for the medical devices and any associated services. It includes the unit prices, discounts (if applicable), payment terms, and invoicing procedures. It may also mention any potential price adjustments due to market fluctuations or regulatory changes. 6. Quality assurance and warranties: The agreement states the quality standards that the medical devices must meet and any warranties provided by the supplier. It may outline the procedure for product inspection, testing, and acceptance at the healthcare facility, as well as the process for handling any faulty or non-compliant devices. 7. Intellectual property: This section addresses the ownership of intellectual property rights related to the medical devices. It defines whether the supplier retains ownership or grants any licenses or rights to the healthcare provider. Different Types of Contra Costa California Medical Device Supply Agreements: 1. Exclusive Supply Agreement: This agreement grants the medical device supplier exclusive rights to supply their products to the healthcare provider within a specific geographic area or for a particular time period. 2. Non-Exclusive Supply Agreement: This type of agreement allows the healthcare provider to procure medical devices from multiple suppliers, without any exclusivity. 3. Long-term Supply Agreement: This agreement establishes a long-term partnership between the medical device supplier and the healthcare provider, usually extending for several years. It provides stability and continuity in the medical device supply chain. 4. Trial or Pilot Supply Agreement: This type of agreement is used when a medical device supplier offers their products for a trial period or pilot project to assess their suitability and effectiveness. It includes provisions for evaluation, feedback, and potential continuation or termination of the supply arrangement based on the trial results.The Contra Costa California Medical Device Supply Agreement is a legally binding contract between a medical device supplier and a healthcare provider in Contra Costa County, California. This agreement outlines the terms and conditions under which the medical devices will be supplied to the healthcare facility. Key Terms and Conditions: 1. Parties involved: The agreement identifies the medical device supplier (company name) and the healthcare provider (hospital, clinic, or healthcare facility) located in Contra Costa County. 2. Definitions: The agreement includes a section that defines the medical devices being supplied, their specifications, and any regulatory requirements that must be adhered to. 3. Scope of supply: This section outlines the specific medical devices to be supplied, including quantity, model numbers, and any additional accessories or spare parts required. It also mentions any limitations or exclusions regarding certain medical devices. 4. Delivery terms: The agreement specifies the terms of delivery, including the delivery location, shipping method, and expected timelines for delivery. It may also outline any penalties or remedies for late or faulty deliveries. 5. Pricing and payment terms: This section details the pricing structure for the medical devices and any associated services. It includes the unit prices, discounts (if applicable), payment terms, and invoicing procedures. It may also mention any potential price adjustments due to market fluctuations or regulatory changes. 6. Quality assurance and warranties: The agreement states the quality standards that the medical devices must meet and any warranties provided by the supplier. It may outline the procedure for product inspection, testing, and acceptance at the healthcare facility, as well as the process for handling any faulty or non-compliant devices. 7. Intellectual property: This section addresses the ownership of intellectual property rights related to the medical devices. It defines whether the supplier retains ownership or grants any licenses or rights to the healthcare provider. Different Types of Contra Costa California Medical Device Supply Agreements: 1. Exclusive Supply Agreement: This agreement grants the medical device supplier exclusive rights to supply their products to the healthcare provider within a specific geographic area or for a particular time period. 2. Non-Exclusive Supply Agreement: This type of agreement allows the healthcare provider to procure medical devices from multiple suppliers, without any exclusivity. 3. Long-term Supply Agreement: This agreement establishes a long-term partnership between the medical device supplier and the healthcare provider, usually extending for several years. It provides stability and continuity in the medical device supply chain. 4. Trial or Pilot Supply Agreement: This type of agreement is used when a medical device supplier offers their products for a trial period or pilot project to assess their suitability and effectiveness. It includes provisions for evaluation, feedback, and potential continuation or termination of the supply arrangement based on the trial results.